
In the process, usable energy is converted into unusable energy. How so? Usable energy is inevitably used for productivity, growth and repair. While quantity remains the same (First Law), the quality of matter/energy deteriorates gradually over time. The Second Law of Thermodynamics is commonly known as the Law of Increased Entropy. Second Law of Thermodynamics - Increased Entropy It can change from solid to liquid to gas to plasma and back again, but the total amount of matter/energy in the universe remains constant. The quantity of matter/energy remains the same. The First Law of Thermodynamics, commonly known as the Law of Conservation of Matter, states that matter/energy cannot be created nor can it be destroyed.

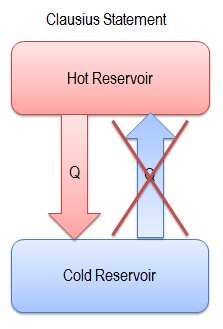
All things in the observable universe are affected by and obey the Laws of Thermodynamics. Thus, the Laws of Thermodynamics are the Laws of "Heat Power." As far as we can tell, these Laws are absolute. The term "thermodynamics" comes from two root words: "thermo," meaning heat, and "dynamic," meaning power. The Second Law of Thermodynamics is one of three Laws of Thermodynamics. Second Law of Thermodynamics - The Laws of Heat Power


 0 kommentar(er)
0 kommentar(er)
